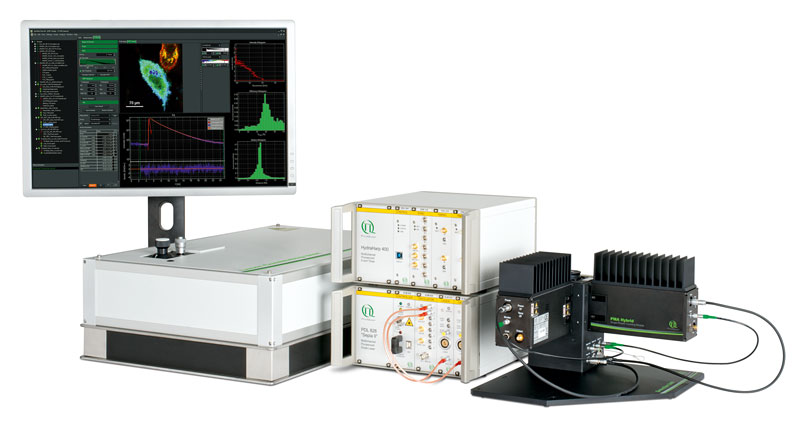
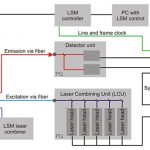
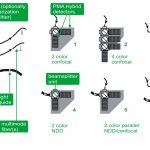
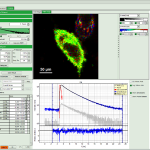
Комплект для апгрейда конфокального микроскопа PicoQuant
Конфокальный лазерный сканирующий микроскоп можно встретить почти в любой биохимической или клеточной лаборатории или ЦКП. Возможности такого микроскопа можно существенно расширить с использованием времяразрешенных методов.
Преимущества:
- Lifetime FRET для количественных измерений эффективности FRET;
- Времяразрешенный имиджинг позволяет визуализировать такие параметры среды как концентрация ионов или pH;
- Время жизни флуоресценции не зависит от концентрации флуорофора;
- Разделение молекул с пересекающимися спектрами эмиссии по временам жизни;
- Сокращение числа необходимых детекторов - одного детектора достаточно для одновременного определения разных флуорофоров на основе их времени жизни, в том числе методом pattern matching;
- Отсечение упругого и рамановского рассеяния от флуоресценции, а также фонового сигнала по времени жизни;
- Время жизни как дополнительный параметр эксперимента повышает точность измерений.
Апгрейд представляет собой набор дополнительных компонентов для микроскопа. Они просты в использовании, универсальны и не требуют большого числа настроек. Базовая установка включает три основных компонента: пикосекундный источник возбуждения, чувствительный к единичным фотонам детектор и устройство коррелированного по времени счета единичных фотонов (TCSPC).
Компактный комплект обновлений FLIM и FCS позволяет использовать многократные приложения с временным разрешением с лазерными сканирующими микроскопами (LSM), например:
- Time-Resolved Fluorescence;
- RapidFLIM — Redefining standards for dynamic FLIM imaging;
- Fluorescence Lifetime Imaging (FLIM);
- Phosphorescence Lifetime Imaging (PLIM);
- Fluorescence Correlation Spectroscopy (FCS);
- Fluorescence Lifetime Correlation Spectroscopy (FLCS);
- Fluorescence Cross-Correlation Spectroscopy (FCCS);
- Foerster Resonance Energy Transfer (FRET);
- Pulsed Interleaved Excitation (PIE);
- Laser Cutting/Ablation;
- Pattern Matching Analysis;
- Time-Resolved Photoluminescence (TRPL);
- TRPL Imaging;
- Antibunching;
- Anisotropy.
| Система возбуждения |
|
| Поддерживаемые LSM |
|
| Обнаружение |
|
| Детекторы |
|
| Сбор данных |
|
| Программное обеспечение |
|
Imaging specific newly synthesized proteins within cells by fluorescence resonance energy transfer
Shen L., Cai L., Liu J., Zhang S., Xu J.-J., Zhang X., Chen H.-Y. Chemical Science, Vol.008, p.748-754 (2017)
Reference to: LSM Upgrade Kit Related to: FLIM, FRET
Silver-coated nanoporous gold skeletons for fluorescence amplification
Lee M.-J., Yang W.-G., Kim J.H., Hwang K., Chae W.-S. Microporous and Mesoporous Materials, Vol.237, p.60-64 (2017)
Reference to: MicroTime 200, SymPhoTime Related to: FLIM
Panek J., Koziolova E., Stepanek P., Etrych T., Janouskova O. Physiological Research, Vol.065, p.217-224 (2016)
Reference to: LSM Upgrade Kit Related to: FLIM
A comparative study of the photophysics of phenyl, thienyl, and chalcogen substituted rhodamine dyes
Sabatini R.P., Mark M. F., Mark D.J., Kryman M.W., Hill J.E., Brennessel W.W., Detty M.R., Eisenberg R., McCamant D.W. Photochemical & Photobiological Sciences, Vol.015, p.1417-1432 (2016)
Reference to: PicoHarp 300, SymPhoTime
Mechanistic determinants of MBNL activity
Sznajder L.J., Michalak M., Taylor K., Cywoniuk P., Kabza M., Wojtkowiak-Szlachcic A., Matloka M., Konieczny P., Sobczak K. Nucleic Acids Research, Vol.044, p.10326-10342 (2016)
Reference to: LSM Upgrade Kit Related to: FLIM, FRET
Structure and dynamics of polyelectrolyte surfactant mixtures under conditions of surfactant excess
Hoffmann I., Simon M., Farago B., Schweins R., Falus P., Holderer O., Gradzielski M. The Journal of Chemical Physics, Vol.145, 124901 (2016)
Reference to: PicoHarp 300, LSM Upgrade Kit Related to: FCS
Doan P. Dissertation University of Michigan (2016)
Reference to: TimeHarp 100/200, LSM Upgrade Kit Related to: FLIM
Control of spontanous emission from quantum emitters using hyperbolic metamaterial substrates
Galfsky T. Dissertation City University of New York (2016)
Reference to: PicoHarp 300, SymPhoTime
Andersen T.G., Nintemann S.J., Marek M., Halkier B.A., Schulz A., Burow M. Scientific Reports, Vol.006, 27766 (2016)
Reference to: MicroTime 200, LSM Upgrade Kit, SymPhoTime Related to: FLIM, FRET
Dakwar G.R., Braeckmans K., Ceelen W., De Smedt S.C., Remaut K. Drug Delivery and Translational Research, Vol.007, p.241-251 (2016)
Reference to: LSM Upgrade Kit, SymPhoTime Related to: FCS